Prof. William Orsi
Professor für Geomikrobiologie
LMU
Sprechstunde:
Wednesday: 14:00-16:00
Postanschrift:
Richard-Wagner-Str. 10
80333 München

The Orsi geomicrobiology lab was started in 2016 in the Department of Earth Sciences at Ludwig-Maximilians-Universität München (University of Munich). As of 2024 the group has produced four PhD students, three postdocs, and more than 13 Master student theses. In addition, a number of international visiting scholars, colleagues, postdocs, PhD students have visited over the years which makes for an active and engaging research environment. We value interdisciplinary science and creative thinking. In all of our projects we value teamwork because we believe that TEAM stands for "Together Everyone Achieves More".
2016-Present Professor of Geomicrobiology (W2), Department of Earth and Environmental Sciences, Paleontology and Geobiology, Ludwig-Maximilians-Universität (LMU) München
2014-2015 Postdoctoral Investigator, Department of Marine Chemistry and Geochemistry, Woods Hole Oceanographic Institution (WHOI)
2013-2014 Assistant Research Scientist, Horn Point Laboratory, University of Maryland Center for Environmental Sciences (UMCES)
2011-2013 Postdoctoral Fellow, Department of Geology and Geophysics, Woods Hole Oceanographic Institution (WHOI) 2006-2011 Ph.D. in Biology, Northeastern University
2002-2006 B.S. in Biology, Temple University
Internal
External
My working group investigates the role of microbial ecology in regulating carbon cycling and greenhouse gas fluxes across marine and subsurface ecosystems. As a geomicrobiologist, my working group focuses on how microbial carbon stabilization processes are linked to microbial greenhouse gas cycling and microbe–mineral interactions. We are interested in the role of microbial diversity in biogeochemical processes at larger scales, and search within the complexity of microbial communities the key microbial taxa for assimilating and transforming elements in ecosystems.
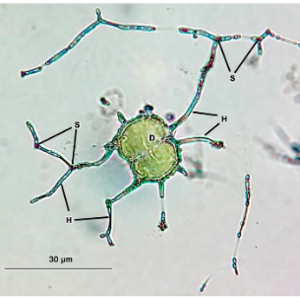
© William Orsi
Microorganisms influence the composition of the atmosphere, the cycling of elements within and through ecosystems, and the functioning of ecosystems. Microorganisms are also the most metabolically flexible, and the most taxonomically and evolutionarily diverse organisms on Earth. Yet deciphering how that diversity influences biogeochemical processes at larger scales is a challenge, because of the overwhelming complexity of microbial communities makes it difficult to quantify how microbial taxa assimilate and transform elements in the environment.
We use a combination of methods that blend traditions from microbial ecology including stable isotope probing, genomic, gene expression, and GC-MS tools to investigate how the diversity and physiology of microorganisms shape carbon cycling of ecosystems.
Our research is derived primarily from field observations, as well as DNA and RNA stable isotope labeling experiments. We are focused on the microbial and biochemical mechanisms underlying the biogeochemical cycling of carbon, and its processing through microbial feeding chains.
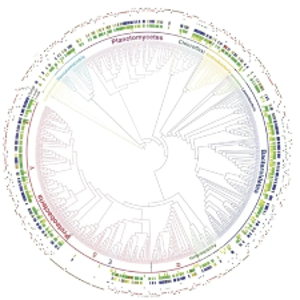
A phylogenetic tree of 16S rRNA genes that were found to correspond to bacteria that assimilation 18O stable isotopes from H218O, identifying population specific growth under anoxia in marine sediments | © Coskun et al., 2019 The ISME Journal
We are interested in deciphering the identify of key microbial groups and or species that are responsible for important C and N cycling processes in nature. To this end, we often use a method called quantitative DNA stable isotope probing that measures the amount of assimilated 13C, 15N, or 18O (stable isotopes) by each and every microbial taxon that is detectable in an environmental sample. Because microbial communities are extremely diverse, this allows us to systematically investigate which microbes are more important than others for assimilation of specific C and N chemicals. Experimental designs allow for controlled testing of hypotheses that can be explored with further experiments or methods. Stable isotope labeling experiments are combined with GC-MS measurements of 13CO2 and 13CH4 to quantify C remineralization rates and estimate microbial carbon use efficiencies.
Trejos-Espeleta JC, Marin-Jaramillo JP, Schmidt SK, Sommers P, Bradley JA, Orsi W* (2024) Principal role of fungi in soil carbon stabilization during early pedogenesis in the high Arctic. PNAS 121 (28) e2402689121.
Orsi W*, Vuillemin, Coskun OK, Rodriguez P, Oertel Y, Niggemann J, Mohrholz V, Gomes-Saez G (2022) Carbon assimilating fungi from surface ocean to subseafloor revealed by coupled phylogenetic and stable isotope analysis. The ISME Journal 16: 1245-1261.
Coskun OK, Vuillemin A, Schubotz F, Klein F, Sichel SE, Eisenreich W, Orsi W* (2022) Quantifying the effects of hydrogen on carbon assimilation in a seafloor microbial community associated with ultramafic rocks. The ISME Journal 16: 257-271.
Orsi W*, Vuillemin A, Rodriguez P, Coskun O, Gomez G, Mohrholz V, Lavik G, Ferdelman T (2020) Metabolic activity analyses demonstrate that Lokiarchaeon exhibitshomoacetogenesis in sulfidic marine sediments. Nature Microbiology 5: 248–255.
Coskun O, Özen V, Wankel S, Orsi W* (2019) Quantifying population specific growth in benthic bacterial communities under low oxygen using H218O. The ISME Journal 13, 1546-1559.
Orsi W*, Richards TA, Francis WR (2018) Predicted microbial secretomes and their target substrates in marine sediments. Nature Microbiology 3: 32-37.

White and green rust chimneys that formed in alkaline vents from chemical garden experiments under ferruginous conditions simulating early Earth ocean chemistry | © Vanessa Helmbrecht
At deep sea low temperature hydrothermal vents chemolithoautotrophic microbes are able to fix CO2 in the absence of sunlight, fueled by geological energy sources. These settings exhibit water-rock interactions and abiotic chemistry called serpentinization, whereby the abiotic synthesis of organic molecules occurs in a naturally exiting proton gradient. This is hypothesized to be analogous to the metabolism of the first cell and the chemolithoautotrophic microbes living at these settings today obtain energy through a similar carbon fixation and bioenergetic pathway. We are interested how this ancient bioenergetic pathway functions in simulated ferruginous environments characteristic of the Archaean and Hadean ocean, its potential role in the origins of life, and the mechanisms by which it supports life and ecosystems. We are able to reproduce "green smoker" hydrothermal chimneys under strictly anoxic conditions, allowing for testing hypotheses surrounding how this primordial metabolism could have supported microbial habitability of early earth habitats and potential exoplanets.
Prüfung der Bewohnbarkeit alkalischer hydrothermaler Schlote in einer simulierten Hadean-Ozeanumgebung (DFG, seit 2023)
Helmbrecht V, Reichelt R, Grohmann D, Orsi W* (2025) Simulated early Earth geochemistry fuels a hydrogen-dependent primordial metabolism. Nature Ecology and Evolution 9, 769-778.
Mills DB, Vuillemin A, Muschler K, Coskun Ö, Orsi W* (2025) The rise of algae promoted eukaryote predation in the Neoproterozoic benthos. Science Advances 11, eadt2147
Helmbrecht V, Weingart M, Klein F, Braun D, Orsi W* (2023) White and green rust chimneys accumulate RNA in a ferruginous chemical garden. Geobiology 21: 758-769.
Weingart M, Chen S, Donat C, Helmbrecht V, Orsi W, Braun D, Alim K (2023) Alkaline vents recreated in two dimensions to study pH gradients, precipitation morphology, and molecular accumulation. Science Advances 9: eadi1884.
Klein F, Humphris SE, Guo W, Schubotz F, Schwarzenbach EM, Orsi WD (2015) Fluid mixing and the deep biosphere of a fossil Lost City-type hydrothermal system at the Iberia Margin. PNAS 112, 12036-12041.

A sediment core taken from the seafloor at the bottom of the Puerto Rico trench, water depth 8550 meters. Note the multiple layers of sediment collected within the core corresponding to sedimentation events into the trench via turbidites. We are studying the role of extreme conditions such as high pressure and energy limitation on microbial life living below the seafloor in this and other deep-sea subseafloor environments. | © William Orsi
We are interested in how life survives under persistent energy limitation, at or close to the energy limits to life, below the seafloor over relatively long timescales (thousands to millions of years). Deep subseafloor and subsurface habitats offer a unique opportunity to study how life can survive over geological timescales without receiving relatively young, freshly produced organic matter from oxygenic photosynthesis and in the absence of higher energy terminal electron acceptors for cellular respiration. Our interest in this topic has led us to investigate microbial communities subsisting in a wide range of energy-limited depositional settings spanning million-year-old deep sea red clay to sandy continental margin sediments. We are particularly interested in understanding how abiotic and biological hydrogen production supports ecosystems at thermodynamic conditions close to energetic limit to life.
Vuillemin A, Kerrigan Z, D’Hondt S, Orsi W (2020) Exploring the abundance, metabolic potential, and gene expression of subseafloor Chloroflexi in million-year-old oxic and anoxic abyssal clay. FEMS Microbiology Ecology 96: fiaa223.
Vuillemin A, Vargas S, Coskun O, Pockalny R, Murray R, Smith DC, D’Hondt S, Orsi W* (2020) Atribacteria reproducing over millions of years in the Atlantic abyssal subseafloor. mBio 11:e01937-20.
Coskun OK, Gomez-Saez GV, Beren M, Özcan D, Günay SD, Elkin V, Hoşgörmez H, Einsiedl F, Eisenreich W, Orsi W* (2024) Quantifying genome-specific carbon fixation in a 750-meter deep subsurface hydrothermal microbial community. FEMS Microbiology Ecology 100(5): fiae062.
Vuillemin A, Wankel SD, Coskun OK, Magritsch T, Vargas S, Estes ER, Spivack AJ, Smith DC, Pockalny R, Murray RW, D’Hondt S, Orsi W* (2019) Archaea dominate oxic subseafloor communities over multimillion-year timescales. Science Advances 5:eaaw4108.
Orsi W* (2018) Ecology and evolution of seafloor and subseafloor microbial communities. Nature Reviews Microbiology 16, 671-683.
Orsi W*, Edgcomb V, Christman G, Biddle J. (2013). Gene expression in the deep biosphere. Nature 499: 205-208.